Background
Throughout history, people have constantly been trying to find more efficient ways to provide food resources to match the demand of an increasing population. They have developed farming systems for various crops, adapting them to the conditions imposed by the specific regions. However, to have high efficiency, in many cases farming systems require a reliable source of large amounts of water. Due to the quantity of water involved, water security holds an essential position in agriculture and is vital for both food production and ecosystem maintenance.
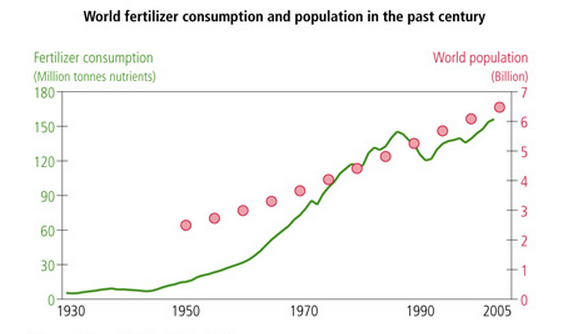
Before the twentieth century, the insufficient amount of nutrients available for crop production was a major factor holding back agricultural expansion and population growth [10]. During the 19th century, Justus von Liebig, a German chemist, proved that a limitation in essential factors, such as nitrogen, water, sun, and salts, could inhibit plants’ growth and, therefore, lower crop productivity [10]. Nitrogen was the hardest one to maintain. This chemical element is scarcely found in minerals because it prefers the more stable, molecular form in the atmosphere, N2. In 1909, Fritz Haber, a German chemist, discovered how to use the molecular nitrogen abundant in air to synthesize ammonia – the main source of the required nitrogen minerals. After this innovation, Carl Bosch, another German chemist and engineer, used fertilizers – organic or inorganic materials added to the soil to supply nourishment essential to the growth of crops, nitrogen being the key element [10]. Fertilizers added to soil proved to be very efficient in providing important nutrients, including nitrogen, for growth. As a result, fertilizer use increased dramatically in the following years. Figures 1 and 2 from bellow illustrate this growth over time.
Figure 1: World fertilizer consumption [11]
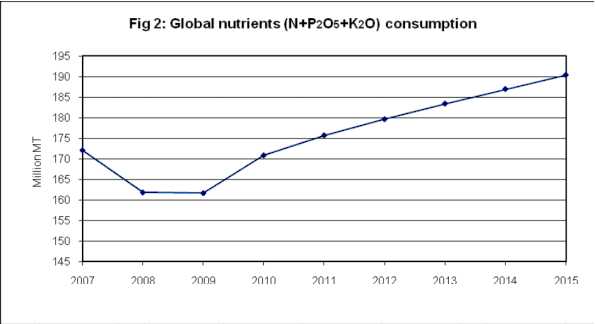
Figure 2: Increase in fertilizer use over the past century [3]
This figure demonstrates the increase in nitrogen and phosphorus fertilizer use over the past decade.
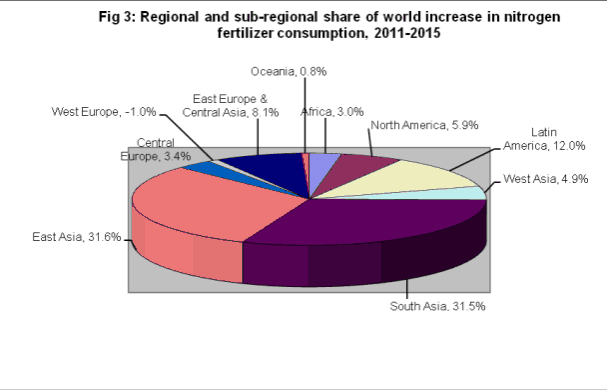
As can be seen in Figure 3, Asia is currently the world’s biggest consumer of fertilizer. Due to a rapidly growing population and an increase in per-capita wealth, concomitant growth in fertilizer use is required to increase agricultural output [3].
Figure 3: Each region and sub-region’s predicted percentage of world increase in nitrogen fertilizer consumption [3]
Due to the growing issue of water scarcity, governments have been forced to reconsider their policies on fertilizer use and take into account both the advantages and disadvantages of fertilizer use. Most found that large-scale efficient food production needs large amounts of both water and fertilizers. Water security requires that the fertilizers used do not contaminate water sources (ex. groundwater, rivers, lakes). It is therefore necessary to fully understand the effects that fertilizers can have on water resources and then seek to minimize them.
Soil Acidification
Poor agricultural practices can cause serious problems for the environment. Prolonged fertilizer application decreases the humus (nutrient organic matter) content of the soil, consequently decreasing the filtration and water retention capacity of the soil [1]. This process occurs because the excessive usage of nitrogen-based fertilizers leads to a surplus of ammonium ions. The excess of ammonium ions contributes to soil acidification [11]. In chemistry, acidity and basicity are measured using the pH scale from 1 to 14. As pH decreases bellow 7, acidity increases. As such, a low pH soil is considered acidic soil. If the soil has low pH, it becomes more vulnerable to erosion [1]. Water leaks more easily through eroded land, so a soil’s filtration and water retention capacity is greatly reduced. Because water cannot be kept near the roots for long enough, plants grow more slowly and can even die. Acidic soil also has a high toxicity level. This implies that acids in the soil destroy important nutrients for plant growth, decreasing the humus content. Therefore, an over-fertilized field can quickly become unusable for agriculture [11].
For example, a long-term fertility trial conducted in Arlington, Wisconsin, revealed that after 30 years of nitrogen-based fertilizers, the soil’s pH was reduced from 7 to 4.8. Also, calcium and magnesium concentrations declined by 31% and 36% respectively. Calcium and magnesium are salts essential for plant growth, therefore such decreases in concentration make the humus almost unusable [12].
How does soil acidification impact water negatively? Farmers use a significant amount of water in the fertilization process. Brian Davidson from Melbourne School of Land and Environment conducted a study on the North China Plain. According to him, “nitrogen applications of between 500 and 600 kg/ha/year are not uncommon, while some farmers have been known to apply up to 800 kg/ha. However, specialists have found that as much as 120 kg/ha more nitrogen fertilizer and 30% more irrigation water was applied each year than was required to obtain an optimal crop yield.” [13]. Farmers are using much more fertilizers than needed, which also requires large quantities of water. Therefore, water security is in danger because of useless waste of resources.
Farmers often apply more fertilizers and water than necessary because of the false belief that it would be more beneficial for crop yields [13]. In an ideal world, the solution would be the introduction of strict governmental policies that limit the amount of chemicals farmers can use on a specific field. However, it is difficult and costly to check that every single person respects the law and conforms to the government’s requests.
The root of the problem lies with a widespread lack of awareness regarding the importance of water scarcity as well as the impacts that fertilizers can have on water resources [13]. The best approach to the implementation of this problem’s solution would require government implication and some financial investments. The first solution would be an educational campaign. Educational specialists employed by governmental organizations responsible for agriculture and water security would talk to farmers directly, explaining the financial benefits of using less fertilizer (lower total expenses), and describing the potential ecological consequences of fertilizer overuse. Having a better understanding of the consequences of bad agricultural practice, more farmers would be able to work according to the policy.
Another possible solution to this problem would be the introduction of governmental tax refunds to stimulate farmers to use less fertilizers and water on their crops. In exchange for a monthly payment, farmers would be more willing to follow the regulations. This solution could be used either alone or together with the educational campaign, according to the funds the government has available. Either way, this idea would give farmers a reason to consider environmental issues in their practices. If applied successfully, the new regulations would prevent soil acidification and result in cleaner water supplies.
Fertilizer Runoff and Eutrophication
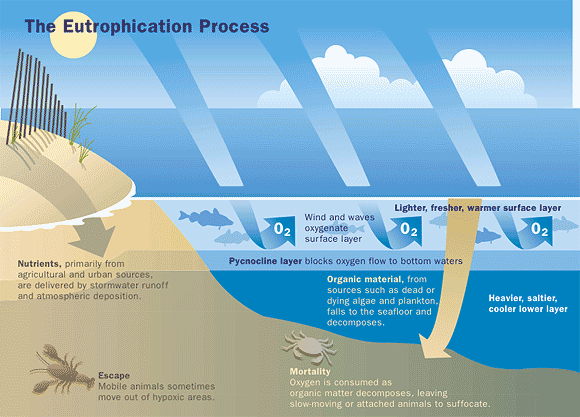
Another imperative issue is that the excess nutrients from fertilizers can run off into the waterways or even contaminate groundwater. The former results in eutrophication, which is the result of an increase of nutrients in water [8]. Such waterway contaminations cause algae to accumulate on the surface. After algae die, they sink to the bottom and decompose. The bacteria decomposing the algae consume the oxygen dissolved in water [8]. Without this indispensable gas fish cannot survive; as such, they either migrate to other areas or die. Figure 4 illustrates this process in more detail.
Figure 4: The eutrophication process and consequences on aquatic beings [5]
Fertilizer runoff puts water security at risk through the process of eutrophication, polluting and poisoning the water supply. An astonishing example that illustrates the level of river pollution is India. In 1984, 5 million tones of fertilizers, 55 000 tons of pesticides, and 125000 tons of synthetic detergents were used in India. Roughly about 25% of all these can be expected to ultimately end up in the rivers every year [14].
Researchers Rashmi Kamble and Dhawal Patil conducted a case study in India, targeting rivers near Pune city. In the past years, they have been testing one innovative solution for eutrophication: the Artificial Floating Island (AFI) technique. [14]
AFI is a low-cost floating structure on which aquatic vegetation grows [9]. Its main purposes are water purification, landscape improvement, and lakeshore preservation. Figures 6 and 7 illustrate the structure of an artificial floating island.
Figure 5: Structure of an artificial floating island [9]
Figure 6: AFI structure and components [23]
Subsystems of the integrated floating island system and demonstration of river water purification in water-net area. (I) Aquatic vegetation near riversides, (II) floating islands with adsorptive biofilms
AFIs are very efficient because the aquatic plants limit algal growth [9]. Aquatic plants have a greater affinity for extra nutrients from fertilizer runoff than algae, so they consume them, limiting the amount algae can use [9]. Not only do AFIs purify river water, but they also provide food and refuge for aquatic animals, creating a habitat for fish and birds. In addition, they increase the aesthetic beauty of the ecosystem. Also, since they are floating systems, they can adapt easily to different water levels and can move from place to place [14]. Therefore, AFIs can be placed in a large number of spaces, including dam lakes with violent waves or changes in water level.
Kamble and Patil conducted two surveys testing floating islands. According to their research, AFI can reduce the Biological Oxygen Demand (BOD – a measure of water pollution) by 60-80% and can also efficiently reduce the Chemical Oxygen Demand (COD – measure of organic compounds in water). AFI can be used efficiently as a solution against fertilizer runoff [14]. They are already increasingly used worldwide, and they are expected to be expanded in developing countries, such as Pakistan or India [9].
Groundwater Leaks
Fertilizers can also be introduced into groundwater through recharge of contaminated runoff. Nitrate contamination of water greater than 45 parts per million can cause methemoglobinemia, a disease that affects livestock and infants [1]. This disease has symptoms varying from dizziness or mental status change to seizures, comas and death. A nested case-control study of methemoglobinemia risk factors in children was conducted in Romania in 2002. The results show there is a strong correlation between the nitrate contamination of water and the number of cases of methemoglobinemia [16].
Also, a great deal of water is used in the food industry and the consequences of the use of nitrate-contaminated water in this sector are potentially disastrous. Nitrates in the food or in the digestive system combine with proteins to form nitrosoamines, which are carcinogenic. This means that people consuming these nitrates have an increased risk of developing tumors that lead to cancer.
Contaminated water is therefore unusable in this form. As more and more water gets polluted, water sources become depleted, contributing to the water scarcity problem. To mitigate the effects of fertilizers on groundwater contamination, specialists analyzed two issues: an excessive use of fertilizers and plants’ low capacity to absorb nutrients.
Richard Cornett, Director of Communications at Western Plant Health Association, reports that, “through nutrient management projects, farmers are implementing BMPs (best management practices) that optimize the efficiency of fertilizer usage by matching nutrient supply with crop requirements and to minimize nutrient loses.” [15]. Specialists are constantly trying to change fertilizing strategies to reduce nitrate leaching in the groundwater. New sensor technologies give the possibility of managing nitrogen and water levels in the ground [17][18]. These sensors use spectral sensing for nitrogen detection and thermal sensing for water stress detection [18]. Electromagnetic energy reflected by vegetation is influenced by the presence of chlorophyll pigments in leaves, which relate to the concentration of nitrogen [18]. By measuring this energy, spectral sensors can give an approximate amount of nitrogen in plants [18]. Also, plants’ temperature can be indicative of differences in water regimes. Plants receiving sufficient water through their roots have cooler leaves than those that are water-stressed. By measuring these temperatures, thermal sensors can predict the amount of water existent in plants [18]. As such, farmers adapt the amount of fertilizers and water they need to use and decide when to apply them based on precise times that can be calculated from the information sensors give [15].
Another increasingly popular solution is fertigation, or irrigation using a solution of soluble fertilizers dissolved in water. The advantage of this technique is that nutrients are better distributed across the fields. With this improved distribution, plants can absorb nutrients with a higher yield, lowering fertilizer and water waste [19]. Combined with the sensor technology, the waste can be further reduced.
Therefore, this solution contributes to water security on multiple levels. First, less water is being wasted because the process of fertigation has higher nutrient absorption rates. The current excessive usage of fertilizers and water gradually decreases in places where these systems are being implemented. Second, a decrease in chemicals use results in less severe groundwater contamination, meaning that more water sources are preserved and usable for consumption.
Conclusion
The identified solutions can greatly contribute to water security. First, farmers need to be stimulated to use the proper amount of fertilizers and water on fields to reduce soil acidification, groundwater contamination, and runoff. Second, artificial floating islands, which are both ecologic and economic, will be used to clean polluted rivers or other surface water sources by using an assembly of plants that consume the nutrients contaminating water. Third, new technologies, such as water and nitrogen sensors or fertigation could help control the levels of added nutrients to increase plants’ capacity to absorb them more efficiently, without an excess.
For the implementation of this solution, the government should first create an independent commission made of two-thirds regular appointed government officials from the agricultural department and one-third appointed specialists in water security in agriculture. Regarding the financial aspect of the solution, the government needs to reallocate the budget in the agricultural department and set a specific amount of money for the Mission 2017 project. Based on the available funds, the commission would then decide how much money to allocate to the soil acidification solution and, according to the circumstances, include the educational program for a limited amount of time or proceed directly with the tax refund system.
The commission first has to analyze their region’s agricultural situation and decide on the new regulations to be implemented. If the budget allows the educational program to be included for a limited time period, it will require the distribution of posters and flyers with all the important educational information on fertilizer use and water security. Special governmental agents would also be sent to the areas with the poorest agricultural management regarding this issue. They would talk directly to the farmers and explain the environmental and economic impacts of their work. The commission would have to analyze the rate of success of this program and decide whether or not to continue using it in combination with the tax refunds system. If it is successful, they could lower the amount of money refunded and reallocate it to another part of the project.
Fertigation would be used in place of normal fertilization and irrigation. The commission would also decide the areas that need AFIs and sensor devices. Afterwards, they would proceed by installing these in the assigned regions; then the devices would be closely monitored by farmers to change the fertigation schedule as appropriate.
By combining the three solutions and correctly following the above-described plan, water security would greatly increase in both cleanliness and amount, as fertilizers would pose a lower threat to our world’s water resources.
References
1. Nicomedes D. Briones, 2005. “Environmentally Sustainable Issues in Philippine Agriculture,” Asian Journal of Agriculture and Development, Southeast Asian Regional Center for Graduate Study and Research in Agriculture, vol. 2(1&2), pages 67-78, June & De
2. Nickum, J. and C. Ogura (2010), “Agricultural Water Pricing: Japan and Korea”, in OECD, Sustainable Management of Water Resources in Agriculture, pages 1-34, OECD Publishing
3. “Current world fertilizer trends and outlook to 2015”, FAO, Rome, 2011
4. Food and Agriculture Organization of the United Nations. “Fertilizer Use by Crop”. Rome: Food and Agriculture Organization of the United Nations, 2006. Web. ftp://ftp.fao.org/agl/agll/docs/fpnb17.pdf
5. “About Eutrophication.” World Resources Institute. N.p., n.d. Web. 10 Nov. 2013. http://www.wri.org/our-work/project/eutrophication-and-hypoxia/about-eutrophication
6. “Coastal Eutrophic and Hypoxic Areas of Asia.” World Resources Institute. N.p., n.d. Web. 10 Nov. 2013. http://www.wri.org/resources/maps/coastal-eutrophic-and-hypoxic-areas-asia
7. “Fertilizer Overuse Damages Agriculture and Environment in China.” The Epoch Times. Epoch Times Staff, n.d. Web. http://www.theepochtimes.com/n2/china-news/fertilizer-overuse-damages-agriculture-and-environment-in-china-62671.html
8. “Chapter 3: Fertilizers as Water Pollutants.” Control of Water Pollution from Agriculture. N.p.: Natural Resources Management and Environment Department, n.d. N. pag. Chapter 3: Fertilizers as Water Pollutants. FAO Corporate Document Repository. Web. 28 Nov. 2013.
9. Fengliang Zhao, S. X. (2011). “Purifying eutrophic river waters with integrated floating island systems.” Elsevier, n.d. Web. 21 Nov. 2013
11. IFA : International Fertilizer Industry Association – A Historical Perspective / Climate Change / Sustainability / HomePage / IFA. N.p., n.d. Web. 21 Nov. 2013.
12. Haynes R J, 1985, “Principles of fertilizer use for trickle irrigated crops”. Fertil. Res. 6, 235–255.
13. Barak P, Jobe BO, Krueger A, Peterson LA, Laird DA., “Effects of long-term soil acidification due to agricultural inputs in Wisconsin” Plant Soil 197:61–69 (1998).
14. Davidson, Brian, and Yong Ping Wei. “Assessing the ‘wicked Problems’ Associated with the Quality of Groundwater Used in Irrigation: A Case Study from the North China Plain.” Hydrogeology Journal Official, Journal of the International Association of Hydrogeologists, 5 Jan. 2012. Web. 21 Nov. 2013.
15. Kamble, Rashmi, and Dhawal Patil. “Artificial Floating Island: Solution to River Water Pollution in India. Case Study: Rivers in Pune City.” N.p., 1 Aug. 2012. Web. 22 Nov. 2013.
16. Cornett, Richard. “Fertilizer Industry Working to Reduce Groundwater Pollution.”Western Farm Press. Western Farm Press, 21 Mar. 2012. Web. 23 Nov. 2013.
17. Zeman, Catherine, Burton Kross, and Marianna Vlad. “A Nested Case–Control Study of Methemoglobinemia Risk Factors in Children of Transylvania, Romania.” Children’s Health 110.8 (n.d.): n. pag. Aug. 2002. Web. 29 Nov. 2013.
18. Sui, R., and J. A. Thomasson. “Ground-based sensing systems for cotton nitrogen status determination.” N.p., n.d. Web. 23 Nov. 2013.
19. Tilling, Adam K. “Remote Sensing to Detect Nitrogen and Water Stress in Wheat.” The Regional Institute. Australian Agronomy Conference, n.d. Web. 23 Nov. 2013.
20. Managing Fertilizers to Reduce Environmental Problems. Agro Services International, n.d. Web. 27 Nov. 2013.
21. Street, M. 1989. Ponds and lakes for wildfowl. Game Conservancy. Technical guidance on constructing and establishing lakes.
22. Bashkin, V. N. “Nitrogen Biochemical Provinces in Central Asia.” Environmental Chemistry: Asian Lessons. Dordrecht: Kluwer Academic, 2003. N. pag. Web. 28 Nov. 2013.
23. Street, M. 1989. “Ponds and lakes for wildfowl. Game Conservancy. Technical guidance on constructing and establishing lakes”, n.d., Web. 28 Nov. 2013