Background
The elemental riches of the earth – from gold to uranium to coal – come at a cost. Mining generates millions of gallons of wastewater and, along with industrial wastewater and sewage, is one of the main sources of water pollution [1].
Mining produces mainly tailings (the waste materials left after the valuable ore is separated out) and overburden (the waste materials removed to reach the ore). During the mining process, water within and around the mine is usually contaminated, so treating the mine water before it is discharged back into the environment is a major issue [2]. For many ores, mineable grades range from the order of a percent to a few ppms. This means that most of the material disturbed in mining is waste: on average, for every ton of copper metal isolated, 99 tons of waste material is produced [3]. It is estimated that over 180 million metric tons of mine waste flow into the world’s waters annually, with devastating results. In the United States, dumping by mining companies into rivers in Montana, Nevada, and Louisiana during the 1970s resulted in some of the most hazardous waste sites in the country that are still being cleaned today. In Japan up to the 1950s, a lead-zinc mining company dumped cadmium-laced tailings into a stream, causing painful kidney and bone illnesses for downriver citizens [4].
Mining results in four main types of pollution: acid mine drainage (AMD), heavy metal pollution, processing chemical pollution, and sedimentation [3]. Chemical waste can severely damage the surrounding ecosystems, as well as poison humans who drink for the water. The effects of mine pollution include liver, kidney, respiratory system, digestive system, and circulatory system diseases, nervous system damage, developmental problems, birth defects, and several types of cancers [5].
Each type of mining presents its own specific problems: coal mining produces coal slurry, a chemical- and coal-particle-laden watery byproduct of washing coal to remove impurities and therefore improve its efficiency when burned. Hydraulic fracturing, or fracking, on the other hand, is the injection of fluid into underground shale to release natural gas deposits, and the waste fluids can create harmful compounds when processed in a typical water treatment plant.
Regulating Mine Pollution with Cap and Trade
National governments should, after creating their own environmental agencies, implement a cap and trade program to reduce mine wastewater pollution. Cap and trade, unlike conventional regulation, will ensure a limit on the total pollutants released into the water. It also creates a source of revenue from selling permits. The government’s environmental agency will review the current levels of pollution and set a cap slightly lower than current pollution output – Mission 2017 suggests a cap at 95 percent of the current level – to be reached five years after being established. In order to reach this level, mining companies can use the technology outlined below. For example, adding an open limestone channel can raise pH by 0.5 of acid mine drainage, bringing the AMD 0.5 closer to the target. These processes are described in detail below.
Regulating Mine Pollution with Restrictions
Cap and trade can control the total amount of pollution discharged, but some practices need to be banned outright. Mission 2017 recommends that the often-occurring practice of dumping tailings, the waste material separated from the ore, into bodies of water is prohibited.
To cut costs, many mining companies employ the practice of sub-aqueous tailings disposal, another word for dumping tailings into oceans, rivers, and lakes, where they poison aquatic life and fisheries, destroying the food supply of those who depend on that wildlife. Ocean dumping, or, in industry terms, “submarine tailings disposal,” causes not only contamination from the chemicals in tailings, but also clouds the water with tailings particles, harming the aquatic life. Additionally, despite mining corporations’ attempts to dump in places where tailings will simply settle, tailings in many sites have been found to spread – at one Canadian site, for example, tailings moved 5 to 35 kilometers past the dumping site. Tailings dumped in rivers through “riverine tailings disposal” can spread contaminants like heavy metals downriver; to floodplains, killing grazing animals; and to private wells, contaminating drinking water. Lake dumping, or “lacustrine tailings disposal,” causes tailings to cover huge stretches of lake bottom, smothering all life there [4].
Cap and trade does not specify where these pollutants can go, only the amount produced. Governments of countries that engage in mining must enforce strict regulations such as banning sub-aqueous tailings disposal.
More information on the danger and alternative storage of tailings can be found in the Chemical Pollution section below.
Achieving the New Standards
As mentioned above, there are four main sources of mine water pollution: acid mine drainage, heavy metal pollution, processing chemical pollution, and sedimentation [3]. In order to reach the new standards set by cap and trade, mining companies can implement a variety of the following methods.
Acid Mine Drainage (AMD)
The process of mining unearths rock that contain sulfides, which react with water and oxygen to form sulfuric acid. The acid is produced as long as there is sulfide present lowering the pH of the water and impacting the surrounding environment by runoff [3]. AMD not only destroys ecosystems when plants and animals are unable to survive in its extreme acidity, but can also dissolve toxic metals, such as arsenic, lead and mercury, from surrounding rocks and mobilize them. Even a small amount of these metals can be toxic to humans and wildlife. Furthermore, AMD often continues indefinitely, as it is impossible to seal the mines from percolating rain water [22].
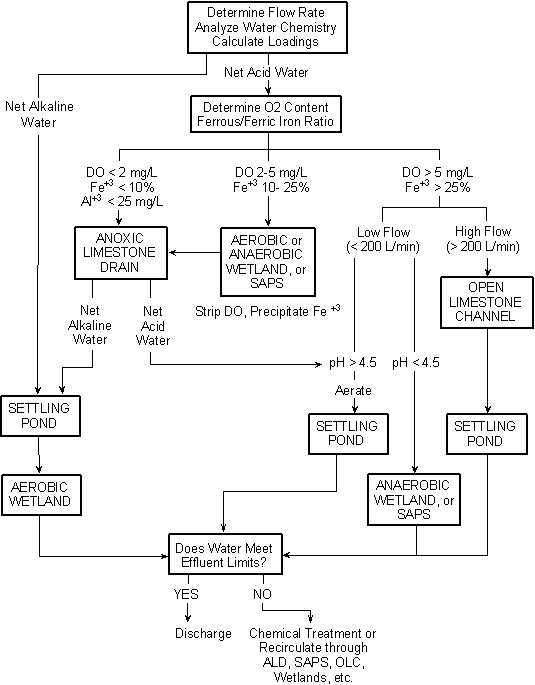
Treating AMD requires neutralizing the acid and precipitating out metal ions. AMD can be treated either with chemicals or through passive systems. Treatment of AMDs relies on the reaction between the basic chemicals and the acidic mine drainage, which produces a neutral metal hydroxide, known as floc, which is a stable material and can be disposed of in abandoned deep mines or landfills [23]. Normal river water has a pH of 7.4; natural water can have a pH from 6.5 to 8.5. AMD should be treated until it reaches a pH of 6.5 [25].
Chemical Treatment
To chemically treat AMD, a chemical treatment system consists of an inflow pipe, a storage tank that holds the treatment chemicals, a way of controlling the application of chemicals, a settling pond where the resulting metal hydroxides will precipitate out as the pH is raised and OH- is added, and a discharge point at which the water should be analyzed to ensure that it is indeed safe. A variety of chemicals can be added to neutralize the acid (Table 1). To oxidize the AMD, chemicals that act as oxidants can be added. Additionally, aeration of the AMD causes oxidation because the oxygen in the air will react with the metals in the water. Oxidation generally allows the metals to precipitate more easily, which increases efficiency and reduces costs. Efficiency improvement depends on the metal; for example, experiments run on the AMD at a Pennsylvania anthracite mine found a 16 to 22 percent oxidation rate in a non-aerated reactor, while the aerated reactor had a nearly 100 percent oxidation rate [23].
|
Common Name |
Chemical Name |
Formula |
Conversion Factor |
Neutralization Efficiency |
1996 Cost |
($ per ton or gallon) |
|
Bulk |
<Bulk |
|||||
|
Limestone |
Calcium carbonate |
CaCO3 |
1 |
30% |
$10 |
$15 |
|
Hydrated Lime |
Calcium hydroxide |
Ca(OH)2 |
0.74 |
90% |
$60 |
$100 |
|
Pebble Quicklime |
Calcium oxide |
CaO |
0.56 |
90% |
$80 |
$240 |
|
Soda Ash |
Sodium carbonate |
Na2CO3 |
1.06 |
60% |
$200 |
$320 |
|
Caustic Soda (solid) |
Sodium hydroxide |
NaOH |
0.8 |
100% |
$680 |
$880 |
|
20% Liquid Caustic |
Sodium hydroxide |
NaOH |
784 |
100% |
$0.46 |
$0.60 |
|
50% Liquid Caustic |
Sodium hydroxide |
NaOH |
256 |
100% |
$1.10 |
$1.25 |
|
Ammonia |
Anhydrous ammonia |
NH3 |
0.34 |
100% |
$300 |
$680 |
Table 1. Chemical Compounds used in AMD treatment [23]
|
Calcium Hypochlorite. |
Ca(ClO)2 |
Strong oxidant. |
|
Sodium Hypochlorite |
NaClO |
Also a strong oxidant. |
|
Calcium Peroxide |
CaO2 |
Trapzene, an acid neutralizer. |
|
Hydrogen Peroxide |
H2O2 |
Strong oxidant. |
|
Potassium permanganate |
KMnO4 |
Very effective, commonly used |
Table 2: Oxidants [24]
To choose chemicals to treat the AMD, first the amount of acid that must be neutralized needs to be calculated. Table 1 shows a few options for chemicals to be used during neutralization [23].
Different chemicals work better under different conditions: for example, under high flow conditions, pebble quicklime and hydrated lime are the most cost effective. Furthermore, AMD from each mine is unique due to the different combinations of acids and metals, and needs to be analyzed so the chosen chemicals are the best tailored to neutralize the specific acids and metals present [23].
In Queensland, Australia, an open pit containing highly polluted mine water – acidic and full of dissolved metals – threatened to spill over into a nearby river. Hydrated lime was added gradually, and as the pH rose, iron, aluminum, and copper precipitated out. Zinc precipitated at a pH of 7.3, and the manganese finally came out at a pH of 9 [10].
Passive Treatment
A less expensive alternative to chemical treatment is a passive treatment system, which might be better suited to developing nations. These take advantage of natural processes and do not require constant application of chemicals. However, they take longer, use more space, and are not as reliable. Nevertheless, they are still a viable low-cost option, well-suited for cash-strapped developing countries. The following are a few examples of passive treatment.
Constructed Wetlands
Constructed wetlands consist of flooded gravel, soil, and organic matter along with wetland plants. Aerobic wetlands, consisting of vegetation in shallow, impermeable soil, are used for alkaline mine water. As the water collects, the metals inside are oxidized by the aerated water, and they settle out. This works on metals such as iron, aluminum, and manganese. Anaerobic wetlands include a layer of limestone in the organic sediment, so they are used with acidic water. In addition to the limestone, bacteria that feed off the organic sediment generate alkalinity to combat the AMD.
Anoxic Limestone Drains (ALDs)
Anoxic limestone drains (ALDs) work by allowing anoxic water (water without oxygen) to flow through buried limestone cells. As seen in the table above, limestone has the properties of a base that can neutralize the AMD. The limestone cells passively produce alkaline bicarbonates that react with the AMD. They work best with deoxygenated water without iron or aluminum present.
The Tennessee Valley Authority currently uses a combination of anoxic limestone drains and aerobic wetlands to treat AMD. The water first goes through an ALD, then a settling basin to hold the iron that precipitated out as the pH rose, and then an aerobic wetland to remove any additional metals. Aerobic wetlands, used in the La Extranjera mine in Spain until its closure, were successful in removing heavy metals from the mine wastewater.
Successive Alkalinity Producing Systems (SAPS)
Successive alkalinity producing systems (SAPS) are a combination of the organic matter found in wetlands and in ALD. This way, the organic matter can consume the oxygen in the AMD so the ALD, which works best with deoxygenated water, can be as efficient as possible.
SAPS are used in Brandy Camp, Pennsylvania, where pH was raised from 4.3 to 7.1, and most of the iron was removed. However, the SAPS were not effective in removing manganese and aluminum.
Limestone Ponds
Limestone ponds are a new form of treatment. The ponds are built over a seep: the seep is covered in limestone, so the water coming out of the seep that fills the limestone pond must pass through (and be neutralized by) the limestone. Their advantage is the ability to see the limestone at work; if the limestone becomes coated with precipitate (thus lowering its efficiency in neutralizing acid), it can be cleaned and returned to full efficiency. They work best with deoxygenated water without iron or aluminum present.
Open Limestone Channels (OLCs)
Open limestone channels (OLCs) are surface ditches lined with limestone. Coating the limestone in iron or aluminum hydroxides (known as “armoring” the limestone) reduces the rate at which it dissolves and lets the OLC last longer. OLCs should be used in conjunction with other passive treatments.
OLCs work best on slopes of more than 20 percent. One OLC built in Brandy Camp, Tennessee on a 10 percent slope raised pH from 4.3 to 4.8, and removed 72 percent of the iron and 20 percent of the manganese and aluminum. Another OLC in Pennsylvania, built on a 45% slope, had a 62 percent decrease in acidity in the form of CaCO3–a decrease from 330mg/L to 125 mg/L.
Diversion Wells
Diversion wells are large vertical tanks filled with grains of limestone. Water flows up through the tank at a rate fast enough to fluidize the limestone, which dissolves, reacts with the AMD, and produces metal flocs that are precipitated out and flushed out the top of the well by the water.
In Western Virginia, a diversion well raised pH from 3.1 to 5.5.
Limestone Dumping
A cruder method involves directly dumping limestone into streams that contain AMD, neutralizing the AMD as the stream flows. An advantage is that the water’s movement keeps the surfaces of the limestone free of accumulated metal hydroxide so the limestone can continue reacting with the AMD [11].
In Western Virginia on the Middle Fork River, four times the amount of the annual acid load was put into the river at various sites; subsequent years will require an amount equal to the annual acid load. Even several miles downstream, water pH was maintained above 6.0. The limestone sand treatment is anticipated to occur three times a year.
Figure 1: Chart detailing process of chemically treating mine wastewater [11]
Heavy Metal Pollution
The metals exposed in mines can be leached out when they come in contact with water, and then are carried out into the environment. This process is especially bad when the water has been acidified by AMD. Metals include arsenic, cobalt, copper, cadmium, lead, silver, and zinc [3]. The most common treatment is chemical precipitation: lime is added to precipitate out metals. This works well with copper, zinc, iron, manganese, nickel, and cobalt. For some metals, more treatment may be necessary at first: for example, chromium requires reduction first with metallic iron before the lime treatment [12]. Treatments for almost every heavy metal present in the wastewater have already been developed but are not always carried out due to costs; once cap and trade has been implemented, with limits on the heavy metals that can be present in wastewater, companies will be forced to utilize these treatments.
Chemical Pollution
Chemicals used by mining companies can also leach out of the mine site into nearby bodies of water. These chemicals are found in the tailings, the waste left over after rock is separated from the ore. Cyanide, which causes heart, brain, and nerve damage [13], and sulfuric acid, which can kill wildlife unable to adjust to the acidity, are two examples of chemicals used to help separate the ore from the mineral [3]. Cyanide and sulfuric acid are used to dissolve gold/silver and uranium, respectively, and then the desired element is then precipitated from the solution [14]. Other sources of chemicals include chemical reagents are used to help flotation separation occur (primarily for the mining of base metal ores, gold, and uranium), where air is introduced into a slurry of ground ore in water. Minerals that are attracted to water remain behind, while those that are attracted to the air attach to air bubbles and float to the top [14].
Tailings can be stored in an appropriate facility; however, the site and construction of the facility must be well analyzed. The site should not be located at a geographically higher point than populated areas, for example, and should avoid delicate ecosystems like wetlands.
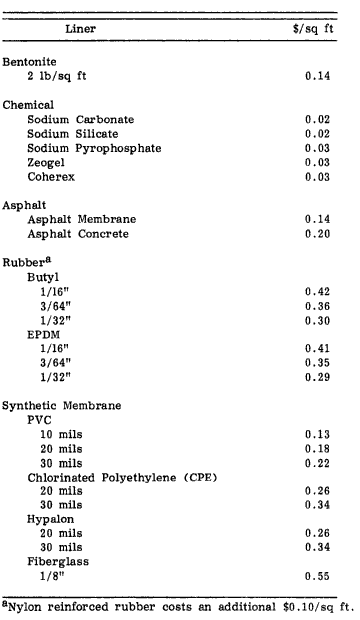
Tailings should be thickened before being sent to the storage facility. Water can be removed through flocculation and settling out, or the addition of chemicals to cause particles to lump together and settle out of the water. Removing water not only lowers transportation costs – estimated at $500,000 per mile [15] – but also reduces seepage once in the facility and provides water that can be reused in the mine. Tailings can be placed in a variety of storage facilities, from pits to inside constructed walls to behind constructed dams. Seepage is an issue that must be considered: if the facility threatens groundwater sources, a clay core or a liner may need to be installed to prevent the underground movement of water. However, liners allow for more extreme storage dams to be built, with steeper slopes and simpler construction methods, which can offset the cost of the liners [16]. In addition, when the mine closes, the costs of cleaning up will be lower if the tailings holders are lined [17].
Table 3: Comparing costs of different liners [17]
Tailings can be covered to protect the surroundings. Vegetation, a thin layer of gravel or combination of the two can be used to shed rainfall runoff and prevent the rainfall from moving through the covered tailings pit to the groundwater.
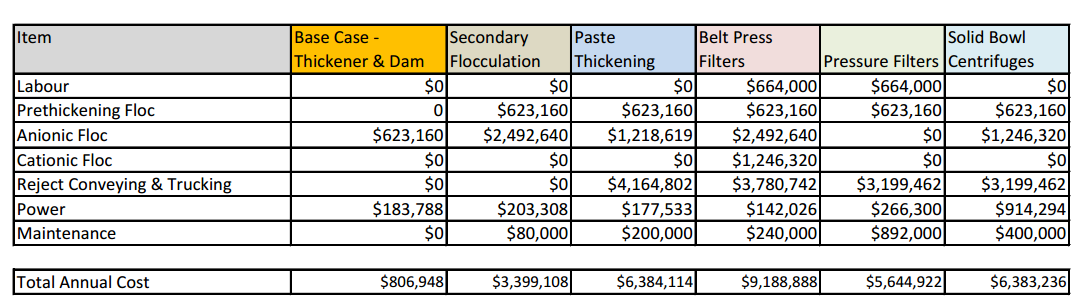
Tailings can also be dewatered by centrifuges and filters in a higher-cost process, but costs can be offset due to the expenses of mines closing due to water shortages, or the costs of cleaning up environmental damage (see Table 3). The leftover tailings “cakes” – chunks of almost waterless tailings – can be moved to storage facilities or used in mine backfilling, the refilling of abandoned mine with tailings [18].
Table 4: Comparing the costs of different methods of dewatering to the conventional thickener [19]
Sedimentation
As roads are built, pits are opened, and waste rock is removed, massive amounts of sediment are exposed to erosion and carried into water bodies. This sediment clogs rivers and chokes up ecosystems, covering vegetation and habitats [3]. A variety of methods can be used to prevent this:
- Routing surface water from mines through sediment ponds slow the flow of water, so it carries less sediment with it.
- Ditches can help direct water movement to keep it away from sites prone to erosion; using rock to build them is the most effective.
- Recontouring the earth: digging troughs parallel along slopes and creating rough ground will slow the velocity of moving runoff so not as much sediment is carried off.
- Revegetation is essential in places of exposed soil, because bare earth has nothing to keep it in place when rains come.
- Silt fences – textiles stretched along stakes – are useful in level areas to prevent movement of sediment.
- Placing impermeable polyethylene sheets on the ground is a temporary solution to erosion [20][21].
Special Types of Mining
Coal mining and fracking are both types of mining, but they present additional problems that can be addressed with the methods described below.
Coal Mining
In addition to the sources of pollution listed above, coal mining produces coal slurry (also known as coal sludge) in the process of beneficiation. The coal is washed with water and chemicals as a way to help it burn more efficiently [22]. The resulting slurry, chock-full of chemicals and coal particles, is usually contained either by placing it into abandoned mines in a process known as injection, where it supposedly is sealed inside underwater pools away from groundwater [22], or dumped into a huge man-made pond, known as a coal slurry impoundment [23]. However, a 2010 West Virginia University study failed to confirm that coal slurry injection does not harm groundwater, a view held by residents in West Virginia, who continue to sue coal companies over claims that slurry has poisoned their wells [22]. Coal slurry impoundments also carry with them the risk of collapse: one disastrous example is the 1972 failure of the Buffalo Creek dam in West Virginia, where over a hundred million gallons of coal slurry broke through the dam’s earthen walls and killed 125 people, injuring hundreds more [23].
However, there are alternatives to injection and impoundment. Dry press filters and dry beneficiation are two new processes that eliminate coal slurry outright.
Dry Press Filters
An alternative to injection and impoundment is dry press filters, which remove the water in the slurry. Though more costly – about fifty cents to a dollar per ton more expensive than conventional treatment plants [24] – the dry waste product is easier and safer to contain [25]. The slurry is placed in a press by batch and pressure is added to produce a filter cake (with less than 25% moisture content) and clarified water, which can be reused [24]. The filter cake is much safer to dispose of and can be placed in a lined landfill.
Dry Beneficiation
Instead of trying to decide what to do with slurry, another alternative is to mine coal without creating slurry at all – that is, removing impurities from coal without using water. New technologies are being developed for dry beneficiation. Even better, dry beneficiation is often cheaper than traditional wet processes [24], and is well-suited to water-scarce regions such as China and South Africa. One example of a recent advance in dry beneficiation is X-ray transmission: the use of an X-ray line-scan sensor to detect and determine whether a material is coal or inorganic material. The sensor gives a rough idea of the atomic number of a particle, and this, combined with a high-resolution image, can even distinguish between different grades of coal. Using X-rays to sort and separate coal from impurities also reduces initial capital required to build the plant because traditional wet processes require more complex infrastructure to handle the large amounts of water and dense coal slurry [26]. Other technologies include using lasers and a particle’s optical characteristics to determine its composition [27]. In China and other water-scarce regions, fluidization is emerging as a viable way to separate coal without water. The coal is placed in a fast fluidized bed, which is a container into which gas is introduced upwards until the gas lifts the particles up, causing the entire bed of particles to act like a fluid, which allows heavier particles to be separated from lighter ones. Dry beneficiation allows the separation of coal from impurities without the use of water, thereby eliminating the problems caused by the creation of coal slurry when coal is cleaned the conventional way, with water [28]. MIssion 2017 recommends dry beneficiation because it not only solves the problem but also cuts out the source of the problem; however, whichever plan is more cost-effective to use should be implemented.
Fracking
Fracking, or hydraulic fracturing, is a process used to collect natural gas from shale deposits. Water, sand, and corrosion-prevention chemicals are injected into underground natural gas wells, creating cracks in the shale that release the natural gas that flows back above ground, where it is collected. For more information on problems related to fracking, as well as detailed solutions that governments should require all fracking sites to implement immediately, visit here.
Financing
As with industry, the burden will be on companies to pay for any upgrades they may have to make. However, no matter the costs of implementing these different types of technology, they are vastly overshadowed by the costs of cleaning up. One example is that of the Coeur d’Alene mining corporation in Idaho, which dumped 56 million metric tons of tailings into a river and a lake that were also contaminated by acid mine drainage. The EPA estimated that a thorough cleanup would last 20 to 30 years and cost $1 billion [4].
Conclusion
In order to prevent further contamination from mines, governments need to implement a cap and trade system to limit pollution from each mine. After cap and trade is established, there are a variety of technologies mining companies can harness to reduce their pollution output, for each of the types of pollution. This can be done to alleviate the pressures mining pollution causes on water. Ultimately, the issue comes down to valuing natural resources properly. If the cost of cleanup is factored into commodity prices, there will be incentive not to leave a long legacy of pollution for others to clean up.
References
1. Tsiho, S. (2007, January 25). Water Pollution in Southern Africa. Gibbs Magazine, 1. Retrieved November 27, 2013, from http://www.gibbsmagazine.com/Water%20Pollution%20in%20Southern%20Africahas%20Gotten%20Bad.htm
2. Doll, B. (2012). Mine Water Treatment Solutions for Discharge and Re-Use. Pall Corporation. Retrieved November 27, 2013, from http://www.pall.com/pdfs/Industrial-Manufacturing/Mine-Water-Treatment.pdf
3. SDWF. Mining and Water Pollution. Safe Drinking Water Foundation. Retrieved November 27, 2013, from http://www.safewater.org/PDFS/resourcesknowthefacts/Mining+and+Water+Pollution.pdf
4. Earthworks (2012). Troubled Waters: How Mine Waste Dumping Is Poisoning Our Oceans, Rivers, and Lakes. Earthworks and Mining Watch Canada, 1, 1-29. Retrieved December 1, 2013, from http://www.earthworksaction.org/files/publications/Troubled-Waters_FINAL.pdf
5. Industrial Mining Activities. (n.d.). Top 10 Worst Pollution Problems 2008. Retrieved December 1, 2013, from http://www.worstpolluted.org/projects_reports/display/60
6. Hardrock Mining: Acid Mine Drainage. Earthworks, 1, 1-2. Retrieved December 1, 2013, from http://www.earthworksaction.org/files/publications/FS_AMD.pdf
7. Skousen, J., Hilton, T., & Faulkner, B. (n.d.). Overview of Acid Mine DrainageTreatment with Chemicals.Overview of Acid Mine Drainage Treatment with Chemicals. Retrieved November 26, 2013, from http://www.wvu.edu/~agexten/landrec/chemtrt.htm
8. Summer Camp 95. (2013, December 1). Water Quality. Water Quality. Retrieved November 30, 2013, from http://www.grc.nasa.gov/WWW/k-12/fenlewis/Waterquality.html
9. Skousen, J., Lilly, R., & Hilton, T. Special Chemicals for Treating Acid Mine Drainage. Green Lands, 34-41. Retrieved December 1, 2013, from http://www.wvu.edu/~Agexten/landrec/AMD_special_chemicals_for_treating_AMD_Su93.pdf
10. Department of Industry, Tourism and Resources, (2007).Managing acid and metalliferous drainage(ISBN 0 642 72512 8). Retrieved from website: http://www.ret.gov.au/resources/documents/lpsdp/lpsdp-acidhandbook.pdf
11. Skousen, J. (n.d.). Overview of Passive Systems for Treating Acid Mine Drainage. Overview of Passive Systems for Treating Acid Mine Drainage. Retrieved November 27, 2013, from http://www.wvu.edu/~agexten/landrec/passtrt/passtrt.htm
12. Dean, J. G., & Bosqui, F. L. (1972). Removing heavy metals from waste water. Environmental Science and Technoloy, 6(6), 518-522. Retrieved November 27, 2013, from http://pubs.acs.org/doi/pdf/10.1021/es60065a006
13. Facts About Cyanide. (2013, June 27). CDC. Retrieved November 29, 2013, from http://www.bt.cdc.gov/agent/cyanide/basics/facts.asp
14. National Pollutant Release Inventory. (2013, July 11). Government of Canada, Environment Canada, Science & Technology Branch, Pollution Data Division. Retrieved November 26, 2013, from http://www.ec.gc.ca/inrp-npri/default.asp?lang=En&n=5D033853-1
15. Branch, S. W. (1994). Design and Evaluation of Tailings Dams. Retrieved December 1, 2013, from http://www.epa.gov/osw/nonhaz/industrial/special/mining/techdocs/tailings.pdf
16. Beck, A., Smith, M., & Sample, K. (2009). Geomembranes for Tailing Impoundments. The Mining Record Retrieved December 1, 2013, from http://www.ausenco.com/uploads/papers/64034_Geomembranes_for_Tailings_Impoundments.pdf
17. Clark, D. A., & Moyer, J. E. U.S. Environmental Protection Agency, Office of Research and Development. (1974). An evaluation of tailings ponds sealants(EPA-660/2-74-065). Washington, D.C.: U.S. Government Printing Office.
18. Australian Government. (2007). Tailings Management. Leading Practice Sustainable Development Program For The Mining Industry, 1-79. Retrieved November 27, 2013, from http://www.ret.gov.au/resources/Documents/LPSDP/LPSDP-TailingsHandbook.pdf
19. Cobbora Holding Company, (2013). Dewatering options report (COB01-J15-N-REP-G-002). Retrieved from QCC Resources website: www.google.com/url?q=http://www.cobbora.com/Resources/Documents/EA/RTS-Report/Appendices/Appendix-C—Dewatering-options-report—comparison-of-options-for-tailings-dewatering.pdf
20. Minerals Education Coalition, (n.d.). Erosion and Sediment Control. Minerals Education Coalition. Retrieved November 27, 2013, from http://www.mineralseducationcoalition.org/reclamation-stories/erosion-and-sediment-control
21. Minto Explorations LTD. (2011). Minto Mine Erosion and Sediment Control Plan. Minto Mine Erosion and Sediment Control Plan, 1, 1-10. Retrieved November 27, 2013, from http://www.emr.gov.yk.ca/mining/pdf/mml_minto_sediment_erosion_control.pdf
22. Smith, V. (2010, August 5). WVU study can’t declare coal slurry injection safe. The Associated Press. Retrieved November 27, 2013, from http://www.businessweek.com/ap/financialnews/D9HDHJ2O0.htm
23. Smith, V. (2012, December 7). Coal Slurry Pond Dangers May Increase As Companies Ignore Construction Standards, Expert Claims. Huffington Post. Retrieved November 27, 2013, from http://www.huffingtonpost.com/2012/12/07/coal-slurry-impacts-dangers-side-effects_n_2255972.html
24. Alternatives. (n.d.). Sludge Safety Project. Retrieved November 27, 2013, from http://www.sludgesafety.org/what-coal-slurry/alternatives
25. What is Coal Slurry. (n.d.). Sludge Safety Project. Retrieved November 27, 2013, from http://www.sludgesafety.org/what-coal-slurry
26. X-ray transmission technology ramps up efficiencies in dry coal beneficiation. (2010, November) Retrieved December 1, 2013 from http://www.tomrasorting.com/mining/news/dual-energy-x-ray-13168
27. Verboomen, J., & Blagden, T. (2009, August). Benchmark for dry beneficiation of coal by laser sorting. Retrieved from http://www.acarp.com.au/abstracts.aspx?repId=C13052
28. Das, M., Saha, R., & Meikap, B. (2010). Hydrodynamic Characteristics of Dry Beneficiation of Iron Ore and Coal in a Fast Fluidized Bed. World Academy of Science, Engineering and Technology , 1, 658-661. Retrieved November 27, 2013, from http://www.waset.org/journals/waset/v41/v41-121.pdf